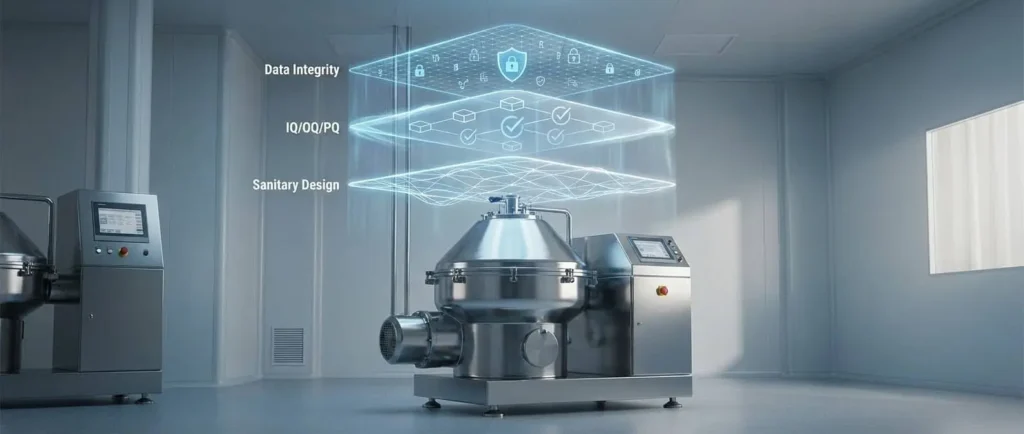
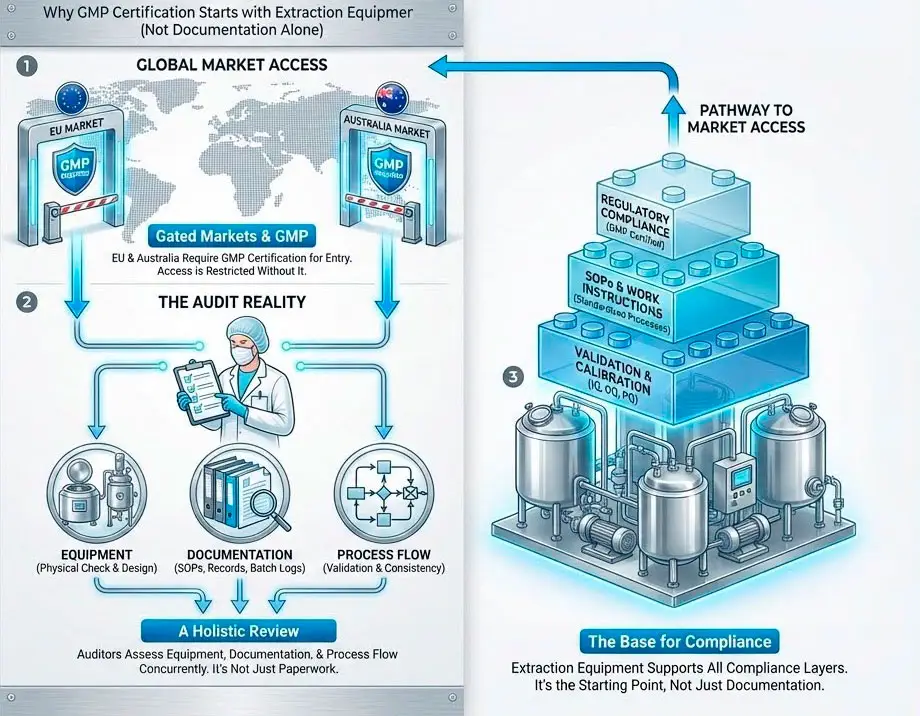
In the current landscape of the hemp and cannabis industry, Good Manufacturing Practice (GMP) certification is no longer a luxury—it is the mandatory entry requirement for the most lucrative global markets, including the European Union and Australia. As production managers, we understand that certification cannot simply be “purchased” with a piece of equipment; it is built through a comprehensive quality system where the hardware serves as the bedrock. The difference between a successful audit and a costly failure lies in the manufacturer’s ability to provide not just a robust machine, but the full traceability and validation documentation that auditors demand.
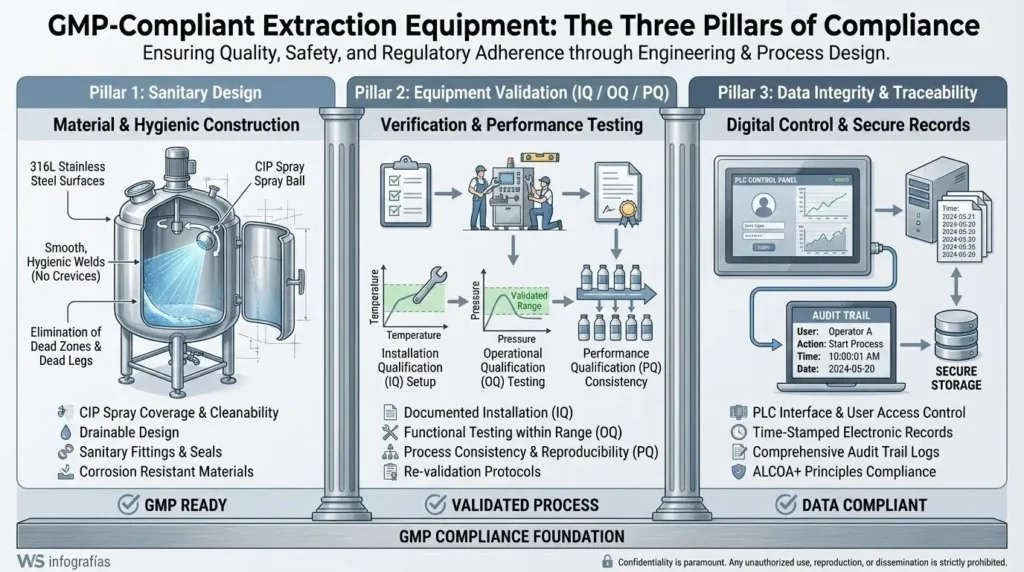
Analysis: The Three Pillars of GMP in Extraction
To reach standards such as EU-GMP or US-cGMP (FDA), extraction equipment must satisfy three non-negotiable requirements: sanitary design, process validation, and absolute traceability.
1. Sanitary Design and Contamination Control
GMP mandates that equipment be designed to prevent cross-contamination and facilitate total cleaning. This requires non-reactive materials, such as 316L stainless steel, and the elimination of “dead spots” where product or microorganisms can accumulate.1 The ability to integrate Clean-In-Place (CIP) systems is critical to ensuring that Standard Operating Procedures (SOPs) for sanitation are repeatable and verifiable.
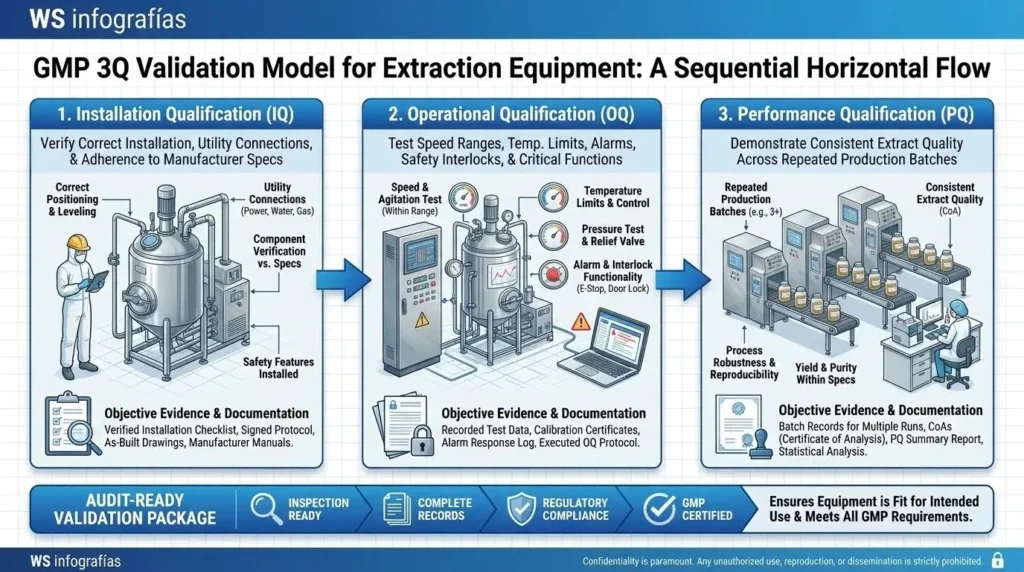
2. Equipment Validation (The 3Q Model)
A GMP auditor will seek objective evidence that the equipment functions exactly as intended. This is achieved through the 3Q validation protocol:
- Installation Qualification (IQ): Verifies the equipment is installed according to manufacturer specifications and supported by proper utilities (electricity, air, nitrogen).
- Operational Qualification (OQ): Demonstrates the machine operates correctly across its entire functional range, including speed and temperature limits.
- Performance Qualification (PQ): Confirms the process consistently produces an extract meeting purity and potency specifications under actual production conditions.
3. Data Integrity and 21 CFR Part 11
In the digital age, GMP requires electronic records to be as reliable as paper. Control systems (PLCs) must include “audit trails” that record who performed an action, when, and why, ensuring that data for every extraction batch is unalterable and traceable.

Strategy with Western States: Your Partner for Global Compliance
Western States Machine Company does more than manufacture centrifuges; it provides a compliance ecosystem designed to mitigate regulatory risk. Unlike equipment adapted from other industries, the WSB (Botanical) line is purpose-built to meet the most rigorous pharmaceutical standards.1
The Technical Advantage of the WSB Series
The WSB-40, WSB-15, and WSB-MicroPro centrifuges integrate engineering features that simplify the path to GMP certification:
| GMP Feature | Western States Specification | Impact on Certification |
| Contact Materials | 316L Sanitary Stainless Steel | Prevents leaching and ensures chemical compatibility. |
| Connections | Sanitary Tri-Clamp | Facilitates easy disassembly and deep cleaning.1 |
| Sanitation | Integrated CIP Nozzles | Ensures 100% verifiable cleaning coverage.3 |
| Area Rating | C1D2 Explosion-Proof | Meets safety regulations for flammable solvent environments.1 |
| Thermal Traceability | Cryogenic Control ($-40^{\circ}C$) | Ensures no thermal degradation during the process.1 |
Validation Documentation: The Deciding Factor
The biggest bottleneck for a production manager is documentation generation. Western States addresses this by providing technical documentation packages that include:
- Mill Test Reports (MTRs): Full traceability of every steel component, ensuring it meets required alloy standards.
- IQ/OQ Protocols: Pre-structured guides that accelerate the validation process at the client’s facility.
- Legendary Dependability: A mechanical structure designed to last decades, reducing the need for frequent re-validation caused by equipment failure.1
Strategic Analysis: Reducing Time-to-Market
Choosing the right equipment directly impacts the certification timeline. A system not designed for GMP from its inception will require expensive modifications and additional testing that can delay commercial production for months.
Consistency and Repeatability via Automation
Variance is the enemy of GMP. Western States’ PLC controls allow for the programming of exact cycle profiles. By fixing parameters such as maximum G-force ($900 Gs$) and retention time, the plant ensures every biomass batch receives identical mechanical treatment.1 This consistency is what auditors look for as evidence of a “process under control”.
Streamlining Processes: “In-Line Winterization”
From an operational standpoint, Western States technology allows for mechanical winterization within the centrifuge by operating at $-40^{\circ}C$.3 By eliminating the need for separate settling tanks and secondary filtration for waxes and lipids, you reduce the number of contact surfaces and Critical Control Points (CCPs) that must be validated in a HACCP or GMP plan.3
Conclusion: Investing in Regulatory Certainty
As production managers, our goal is efficiency without compromising product integrity or consumer safety. GMP certification is the ultimate guarantee of that integrity. Western States offers more than a high-efficiency centrifuge; it offers a validated compliance platform backed by a century of experience in the chemical and pharmaceutical sectors.5
By integrating Western States equipment, a facility does not just optimize solvent recovery to 97%+; it gains the peace of mind that its infrastructure meets the expectations of the world’s most demanding auditors.1 In a market rapidly shifting toward federal and pharmaceutical regulation, partnering with Western States is the strategic decision to future-proof your operation.
Obras citadas
- High-Performance Botanical Centrifuges for Botanical Extraction – Western States, fecha de acceso: diciembre 29, 2025, https://www.westernstates.com/high-performance-botanical-centrifuges-for-botanical-extraction/
- Quadramatic – Western States | Centrifuge, fecha de acceso: diciembre 29, 2025, https://www.westernstates.com/quadramatic/
- The Cold Standard: Unlocking Superior Terpene Retention with Cryogenic Centrifuge Technology – Western States, fecha de acceso: diciembre 29, 2025, https://www.westernstates.com/the-cold-standard-unlocking-superior-terpene-retention-with-cryogenic-centrifuge-technology/
- Centrifuges – Western States – Western States | Centrifuge, fecha de acceso: diciembre 29, 2025, https://www.westernstates.com/centrifuges/
- Western States | Centrifuge Manufacturers in the USA for Chemical & Pharma – Western States, fecha de acceso: diciembre 29, 2025, https://www.westernstates.com/
- Types of Sugar Centrifuges: Batch vs. Continuous – Western States, fecha de acceso: diciembre 29, 2025, https://www.westernstates.com/batch-vs-continuous-centrifuge/
- WSB-40 – Western States | Centrifuge, fecha de acceso: diciembre 29, 2025, https://www.westernstates.com/wsb-40/
- The Cold Standard: How Low-Temperature Ethanol Extraction Preserves Cannabinoids and Terpenes – Western States, fecha de acceso: diciembre 29, 2025, https://www.westernstates.com/the-cold-standard-how-low-temperature-ethanol-extraction-preserves-cannabinoids-and-terpenes/
- Chemical And Pharmaceutical Centrifuges – Western States, fecha de acceso: diciembre 29, 2025, https://www.westernstates.com/chemical-and-pharmaceutical-centrifuges/